- Dec 10, 2025
- By My Store
- 0 comments
How FDA Flavor Rules May Reshape the U.S. Vape Market in 2025
How FDA Flavor Rules May Reshape the U.S. Vape Market in 2025
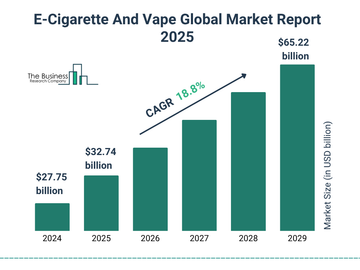
Flavors are central to the U.S. vaping debate and continue to shape regulatory decisions in 2025. As FDA intensifies enforcement around flavored disposables, wholesalers and retailers must prepare for significant industry shifts.
1. Why Flavors Are Under Regulatory Pressure
The FDA views flavored products — especially fruit, candy, and dessert profiles — as potentially appealing to underage users. As a result, these SKUs face the highest enforcement and denial rates during the PMTA review process.
Adult users, however, overwhelmingly prefer flavored vapes over tobacco-flavored ones, creating a challenging dynamic between regulation and consumer demand.
2. Impact on Disposables
Disposables are the primary targets of flavor enforcement. FDA import alerts and retail crackdowns often focus on fruit-flavored and non-authorized models.
Risk factors include:
- Non-PMTA fruit flavors
- High-nicotine synthetic blends
- Unverified manufacturing origin
Retailers in strict states may lose access to many flavor-based SKUs entirely.
3. Shifting Market Dynamics
If FDA introduces new limitations, the market may experience:
- Growth in tobacco and menthol flavor lines
- Increased demand for nicotine-free flavored alternatives
- A return to refillable pod systems, where flavors may face fewer restrictions
- Emergence of compliant “adult flavor” categories (e.g., botanical, herbal, beverage-inspired)
Many manufacturers are already preparing alternate formulations designed to meet regulatory expectations.
4. What Distributors Should Prepare For
To stay ahead of market changes, distributors should diversify their catalog and create product portfolios resilient to regulatory shifts.
Recommended strategy:
- Offer strong tobacco/menthol selections
- Introduce 0% nicotine flavored SKUs
- Partner with PMTA-applied brands
- Educate retail customers on compliant product categories
Whether the FDA tightens or stabilizes flavor rules, distributors who prepare now will be positioned to succeed in any scenario.